Studies
A Retrospective Review of IUI Clinical Outcomes Following Semen Collection in the ProteX versus a Standard Specimen Cup
Preliminary Clinical Outcomes in an IVF Program using the ProteX versus a Standard Specimen Cup for Semen Collection
Early Fertility Trials of Semen Collection Device Previously Demonstrated to Improve Semen Parameters and Pregnancy Rates in Animal Models
Pregnancy Trials Using the Device for Improved Semen Collection
Physiological and Biochemical Assessment of a New Semen Collection Device
Continued Evaluation of a New Semen Collection Technique / Container in Subfertile and Infertile Individuals Using a Cross-species Model
New Semen Collection Technique / Container Improves Semen Quality
Improving Semen Quality Using a Modified Collection Technique
A Novel Collection Technique for Improved Semen Quality
Improvement of the Semen Collection Environment Using a New Semen Collection Device
ProteX Original Research | Animal Study
A Novel Collection Technique for Improved Semen Quality
Authors
- Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX
- Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, Lubbock, TX
Publications
Objective
To date, the common thread in the use of semen extenders / collection techniques, whether they are being used for fresh extended semen, chilled semen, or cryopreserved semen, is that the extenders have all traditionally been added post-collection. The objective of this study was to determine if modifying the method of collection / extension of semen to include a warmed media environment would improve semen parameters by lessening cold and pH shock.
Materials and Methods
Ten canine semen samples were collected with a modified artificial vagina to allow for a true split collection into two collection containers at one time. The treatment half of the sample was collected into a measured amount of warmed extension media. The control half was collected into a dry container and no attempt to maintain temperature was used. Standard semen parameters, available sperm pool, and number of inseminations were evaluated at specific time intervals. Evaluations continued until samples reached zero percent motility. Data analysis was performed with SPSS using the general linear model and appropriate t-tests.
Results
Conclusions
Modification of the semen collection / extension procedure resulted in improved semen parameters for extended time periods post-collection. The data suggest the described collection technique can yield significantly more motile sperm by placing the sample into a physiologically favorable environment (eliminating pH and cold shock and allowing osmoregulation to begin), thus providing more available sperm for breeding.
Insights
This is the original canine study and is the first to describe the concept of a modified collection method, Device for Improved Semen Collection (DISC), that would eventually become ProteX. Canines were chosen as the initial model because canine sperm are one of the more difficult to maintain for any length of time outside the body. In addition, canine sperm have similar volume, concentration, and sperm physiology to humans. Unlike other animal models, there are infertile canines, creating another correlation to human diagnoses.
Prior research by the investigators determined that the specific amount of media to be used in the new device prior to collection should be ~20% by volume of the expected ejaculate. This ensures the sperm are osmotically balanced. In the case of canines and humans, media should be 1 mL. This amount was determined to be optimal for providing the pH buffering capacity and allowing the sample to begin osmoregulating, without causing osmotic stress.
Modified Artificial Vagina
The modified artificial vagina was developed by the investigators to allow for a true split collection as opposed to a fractionated collection. This allowed for a direct comparison of the DISC to traditional collection using the same ejaculate.
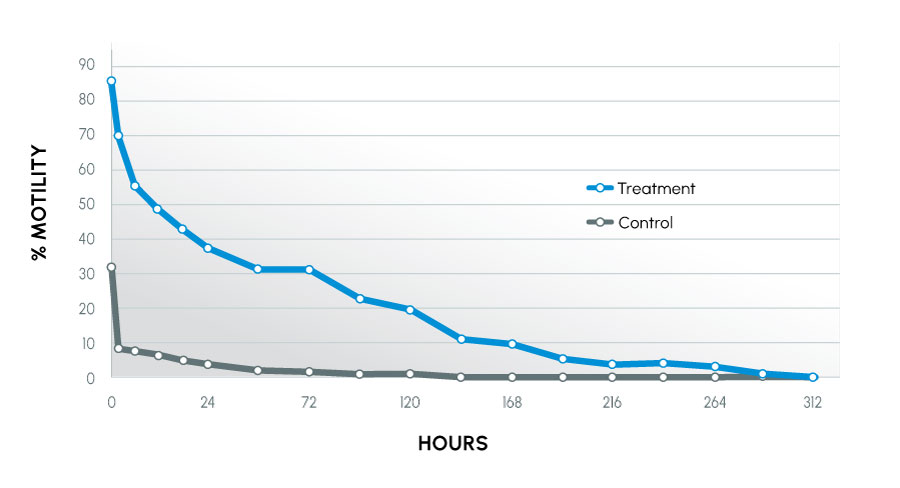
Figure 1
Motility Over Time – all canines
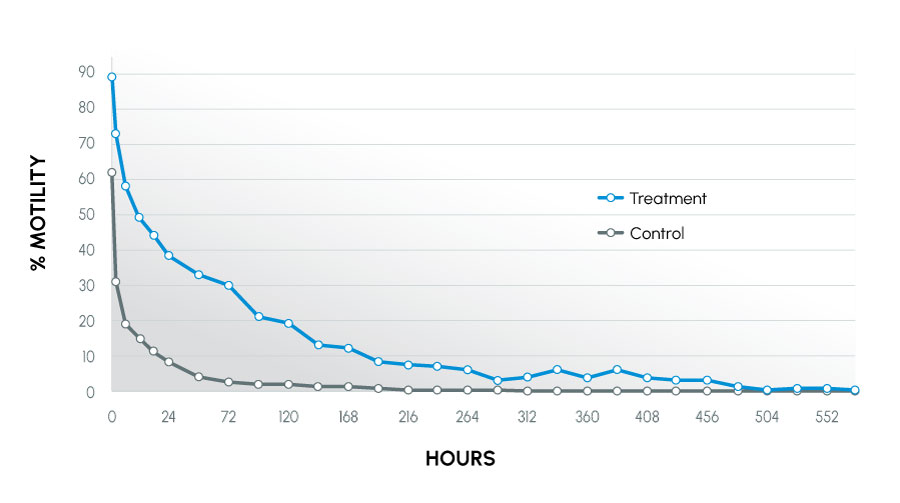
Figure 2
Time to Last Full Insemination Dose – canines
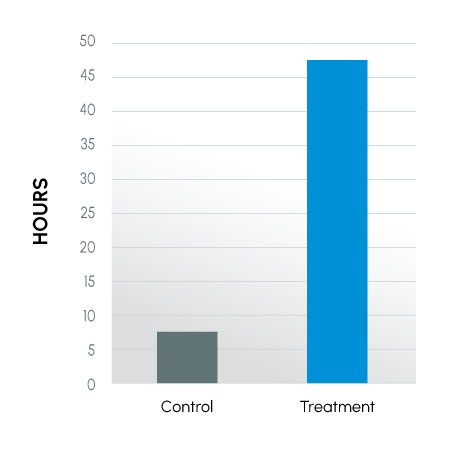
Figure 3
Motility Over Time tolerant canines
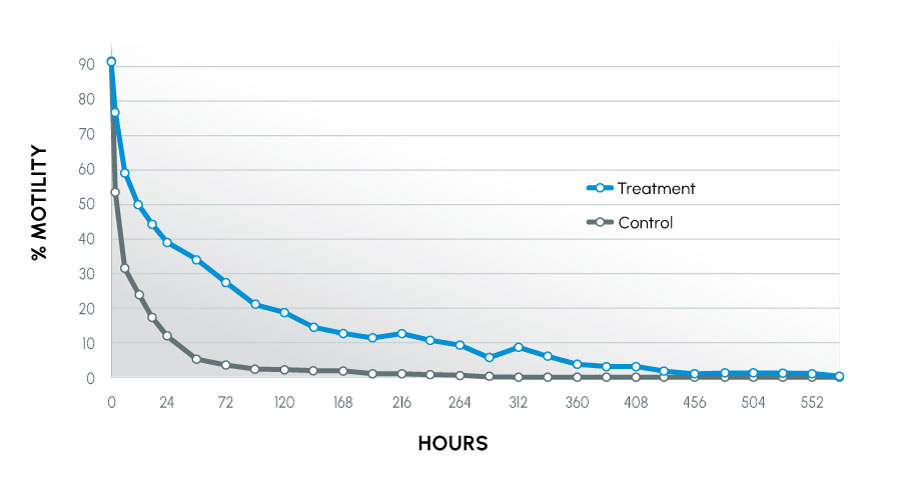
Figure 4
Motility Over Time intolerant canines
Method Tolerance and Acrosomes
Animals used in the study were recognized as being tolerant (normal) or intolerant (infertile) to traditional collection methods. The animals that were intolerant to traditional methods had the most improvement. This study also showed that more motile, acrosomally intact sperm were maintained in the treatment group. The result indicated sperm better maintained their fertilizing capability.

Acrosomally Intact
Sperm
Acrosomally Reacted
Sperm
Direct insights into the research, methodology, and results have been added to this summary by the co-inventors themselves. This additional information is intended to provide helpful context to professional practitioners and does not fundamentally change the outcomes or interpretation of the published results. All ProteX research content and material is the property of Reproductive Solutions and may not be redistributed or republished without our consent. All rights reserved.
