Continued Evaluation of a New Semen Collection Technique / Container in Subfertile and Infertile Individuals Using a Cross-species Model
Authors
Samuel Prien1 2, Dustie Johnson1, Clay Dehn1
- Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX
- Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, Amarillo, TX
Publications
Fertility and Sterility Vol. 82, Supplement S178. Published in issue: September 2004.
Objective
To date, the common thread in the use of semen collection / extension techniques, whether being used for intrauterine insemination, cryopreserved semen, or advanced reproductive techniques such as in vitro fertilization, is that the samples are collected into a dry, unprotected specimen container. This container, by its nature, allows for drastic shifts in temperature and pH resulting in damage to the spermatozoa.
Preliminary studies from this laboratory using a new collection container / technique to collect semen from canines and humans demonstrated an improvement in most semen parameters. The modified collection device optimizes semen parameters by controlling temperature, pH, and osmotic stress. The objective of the current study was to further investigate the usefulness of the modified device in improving semen parameters using a cross-species model focusing on subfertile and infertile individuals.
Materials and Methods
Semen samples were collected from canine (n = 10), equine (n = 8) and human (n = 12) donors using standard techniques. In the case of the equine and canine donors, a splitter was placed in the artificial vagina to produce a true split sample into a standard collection vessel and the modified collection unit.
Human donors were collected into both devices in sequential samples at three-day intervals. Standard semen parameters (viability, motility, morphology, concentration) and biochemical markers were evaluated for all donors at 0, 1, 3, 6, 12, 18, and 24 hours post-processing. Canine and Equine samples were further evaluated at 24-hour intervals until the samples had reached 0% motility. All donors were then classified as either fertile or subfertile based upon species standard for count and / or motility. Data analysis within species were performed with SPSS using the general linear model and appropriate t-tests.
Results
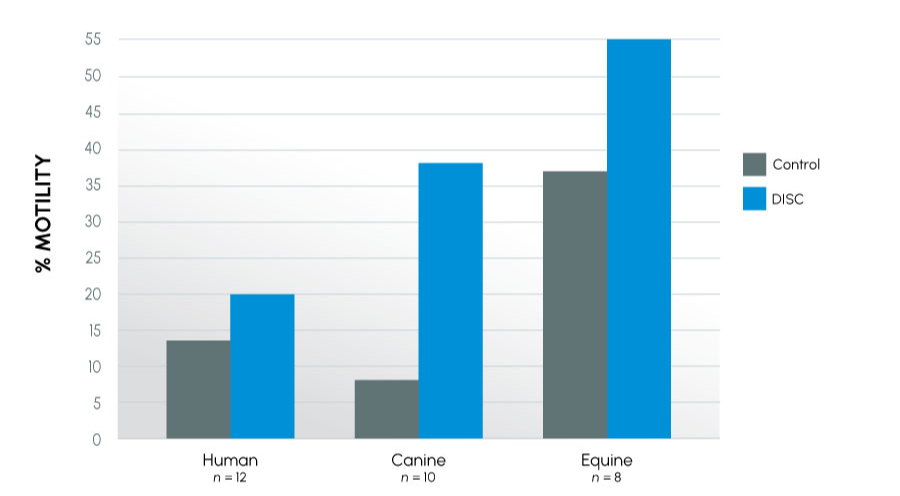
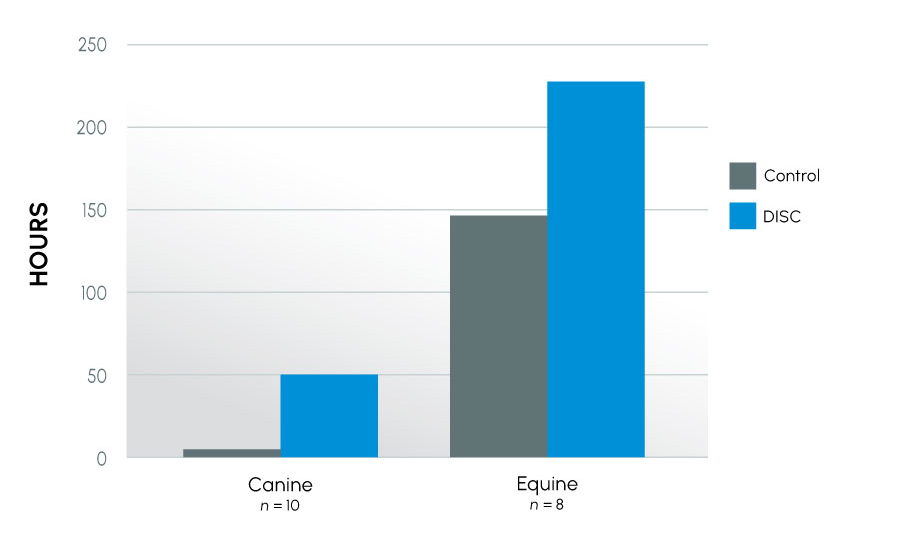
As in previous studies, semen quality at 24 hours (as reflected by motility) was significantly higher in samples collected in the new collection container as compared to the control regardless of species: human 20% vs. 14% (p < 0.001); canine 38% vs. 8% (p < 0.001); equine 55% vs. 37%, (p < 0.001). Further, collection of the samples into the new device extended the time to last full insemination dose an average of 6-fold (7 hours vs. 50 hours) in the canine (p < 0.03) and 0.5-fold (148 hrs vs. 228 hrs) in the equine (p < 0.001) over their respective control. This improvement was even more dramatic in animals classified as subfertile, where collection into the new device extended the time to last full insemination dose an average of 14-fold (6 hours vs. 91 hours) in the canine and 1.5-fold (120 hours vs. 312 hours) in the equine over their respective controls. Improvement was also seen in viability and biochemical parameters.
Conclusions
Modification of the semen collection / extension procedure resulted in improved semen parameters for extended time periods post-collection. The data suggests the described technique can yield significantly more motile sperm by placing the sample into a physiologically favorable environment (eliminating pH and cold shock), thus making more sperm available for use in a variety of infertility treatments. Further studies, evaluating pregnancy rates, will be needed to confirm these observations.
Figure 1
Motility Using the Disc – Cross-Species View at 24 Hours
A commercial trial of conception rates in cattle using semen collected in the DISC (Treatment) vs. industry standard collection techniques (Control). Data suggests higher pregnancy rates from semen collected in DISC (p < 0.02).
Insights
In this study, the twelve human subjects were all diagnosed as subfertile with six subjects providing a unique sample in each arm. Note the human motility at 24 hours for both Control, DISC, and the resulting delta differs from a previous motility study. This variance is due to the small sample size, starting motility, and the diagnosis of this group. Average starting motility for this sample was ~20%, and therefore nearly flat through the 24-hour mark. This coincides with previous trends that indicate a long-term stability of samples in the DISC.
Figure 2
Time to Last Full Insemination Dose – Subfertile Canine and Equine
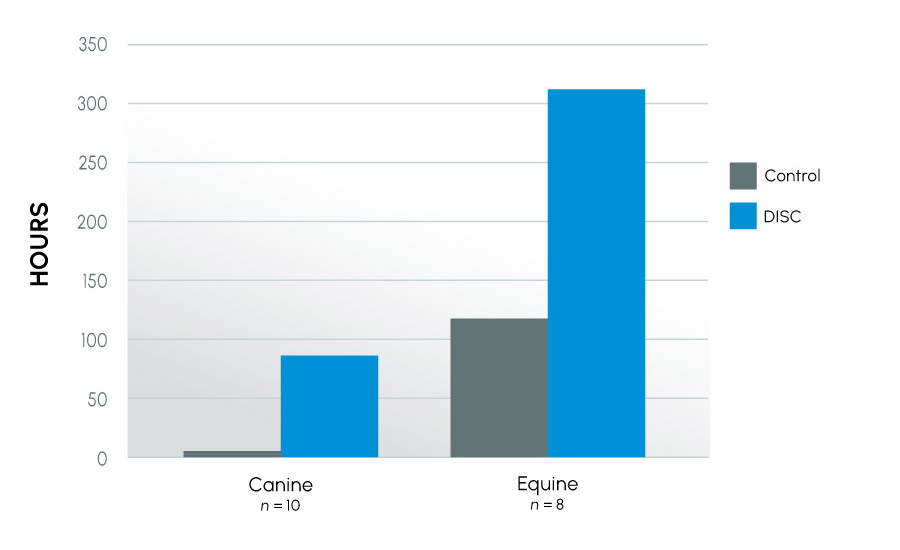
Figure 3
Conception Rates Over Time – EquineTime to Last Full Insemination Dose – All Canine and Equine
Insights
This series of experiments examined how semen from different species reacted in ProteX. The species evaluated included human, equine, and canine, all species that can have infertility. Both fertile and infertile canine and equine subjects were used, as well as subfertile human subjects. Motility was improved in all species, further, time to last full insemination dose was significantly improved in both canine and equine models. The greatest improvement was seen in subfertile animals. This indicates that protecting the sperm from damage at collection has the greatest impact on the infertile male.
Direct insights into the research, methodology, and results have been added to this summary by the co-inventors themselves. This additional information is intended to provide helpful context to professional practitioners and does not fundamentally change the outcomes or interpretation of the published results. All ProteX research content and material is the property of Reproductive Solutions and may not be redistributed or republished without our consent. All rights reserved.
All Scientific Studies
Sample. Test. See for yourself.
Compare ProteX to the standard specimen cup with our sterile sample kit.