Studies
A Retrospective Review of IUI Clinical Outcomes Following Semen Collection in the ProteX versus a Standard Specimen Cup
Preliminary Clinical Outcomes in an IVF Program using the ProteX versus a Standard Specimen Cup for Semen Collection
Early Fertility Trials of Semen Collection Device Previously Demonstrated to Improve Semen Parameters and Pregnancy Rates in Animal Models
Pregnancy Trials Using the Device for Improved Semen Collection
Physiological and Biochemical Assessment of a New Semen Collection Device
Continued Evaluation of a New Semen Collection Technique / Container in Subfertile and Infertile Individuals Using a Cross-species Model
New Semen Collection Technique / Container Improves Semen Quality
Improving Semen Quality Using a Modified Collection Technique
A Novel Collection Technique for Improved Semen Quality
Improvement of the Semen Collection Environment Using a New Semen Collection Device
ProteX Clinical Trial | Human Study
A Retrospective Review of IUI Clinical Outcomes following Semen Collection in the ProteX versus a Standard Specimen Cup
Authors
Samuel Prien1, Zev Williams2, Eric Forman2.
- Reproductive Solutions, Dallas, TX
- Columbia Center for Fertility, Columbia University, NY, NY
Publication
American Association of Bioanalysts Conference. May 2022 – poster presentation.
Objective
While intrauterine insemination (IUI) using freshly ejaculated, prepared semen combined with ovarian stimulation of the female partner remains a first-line treatment for infertility, it has a relatively low success rate (approximately 15% per cycle). An obvious rate-limiting step in the success of the process is the quality of the male partner’s semen.
Traditionally, clinics have used a standard specimen cup (SSC) for specimen collection, a cup designed for 25-150 mL of urine, not the 2-5 mL of a standard semen sample. The objective of the present study was to compare a new sperm collection device (ProteX), explicitly designed to maximize available sperm by providing a collection environment that supports sperm function, to the SSC.
DESIGN
Retrospective study of IUI cycles in which a NSCD was compared with those using the SSC.
Materials and Methods
Retrospective data from 338 patients undergoing IUI for six months after the clinic switched semen collection device to the ProteX were compared to the previous six-month period using the SSC. All samples were treated using standard lab procedures post-collection. Data collected included male and female partner’s age, female partner’s method of ovarian stimulation, initial and post-processing semen parameters, including volume, concentration, motility, and total motile count.
Results
Female partners ranged in age from 21-48, with means of 34.8 vs.35.4 (SSC vs. ProteX, respectively). In contrast, there were differences between the groups for method of ovarian stimulation. However, the majority of patients in each group were treated with Clomid or Letrozole (> 80%). Males’ ages were similar (36.7 vs. 36.8). However, men collecting in the ProteX had higher volumes (2.2 VS. 2.5 mL; P < 0.008), motile cells (48.3 vs 53.0 mil; P < 0.001) and total motile counts (73.0 vs 91.3; P < 0.005). While both groups had initial pregnancy rates of 12%, 90% of the pregnancies resulting from sperm collected in the ProteX continued to heartbeat versus 70% in the SSC (P < 0.02).
Conclusions
This retrospective study suggests that ongoing pregnancy rates may be improved by producing semen samples in a more physiologic collection container with a measured amount of culture media (ProteX), that is designed to provide healthier cells for reproductive procedures. These findings agree with earlier animal studies.
FUNDING
ProteX were provided by Reproductive Solutions.
DISCLOSURES
Reproductive Solutions supplied all ProteX for use in this retrospective review. Samuel Prien is an inventor of the technology, a consultant to Reproductive Solutions, and a stockholder in Reproductive Solutions.
Figure 1
Design and concept of the device
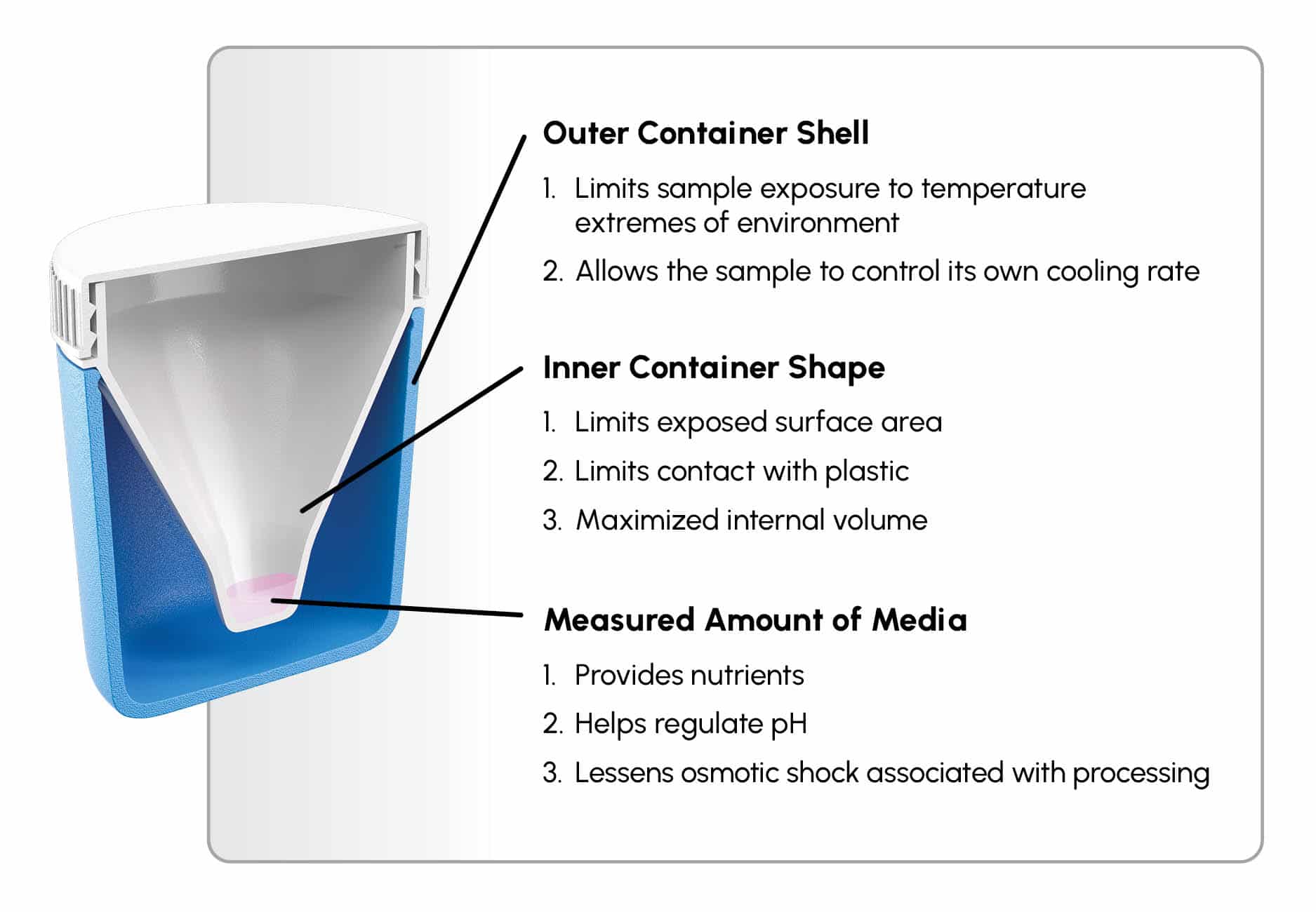
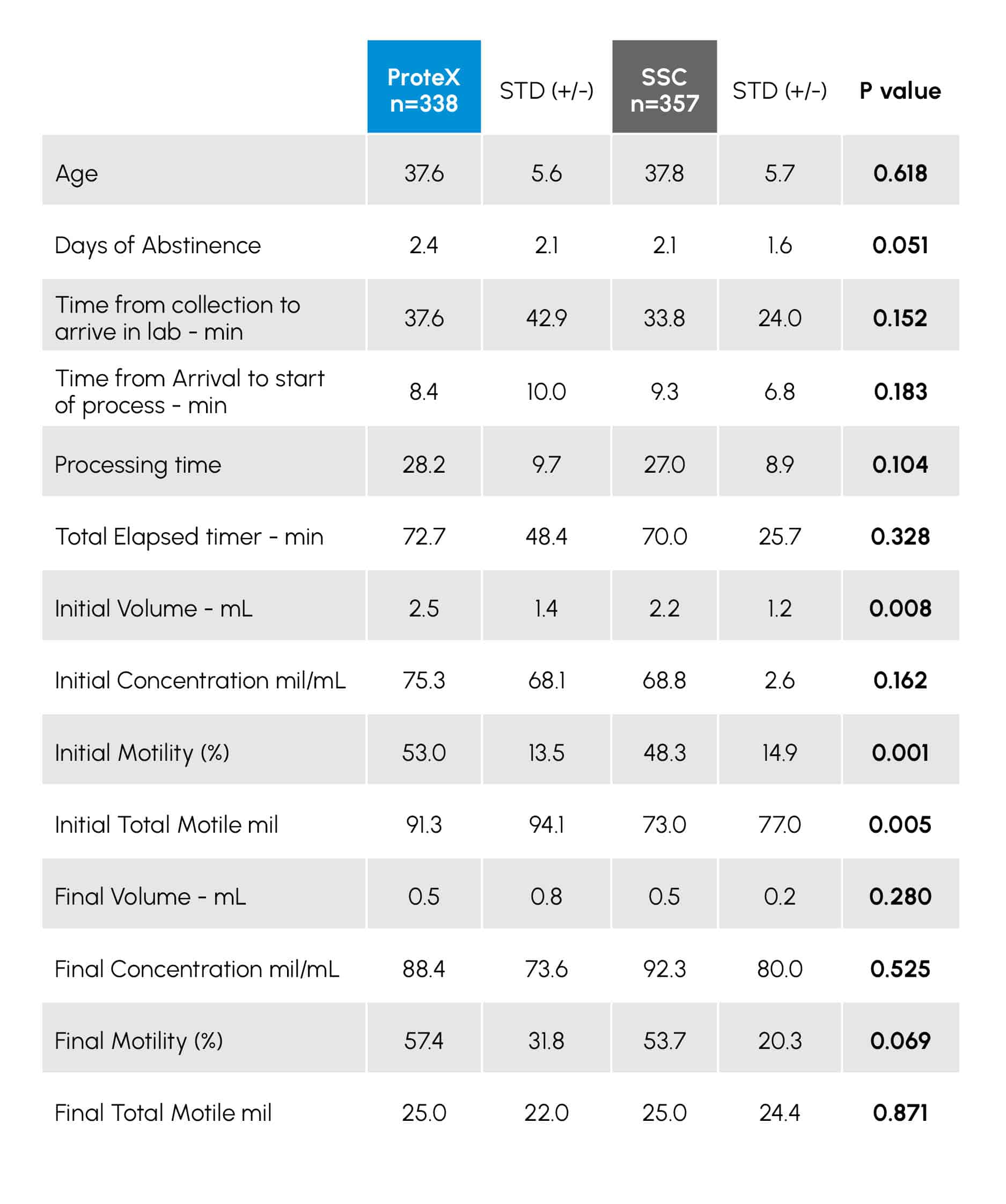
Table 1
Demographic data and semen parameters from the review of 695 cycles using the ProteX (n=338) or Standard Specimen Cup (SSC; n=357) for semen collection and use in IUI. Data with a P value of < 0.05 are considered statistically different.
Insights
Patients were given the option of collecting in the clinic or at home. While data on location was not given, the average time between the collection and start of processing was similar in both groups (TABLE 1; P = 0.183), suggesting equivalent numbers in both groups collected in both locations. TABLE 1 provides the demographic data from the male patients. These data were similar between both groups (P = 0.681).
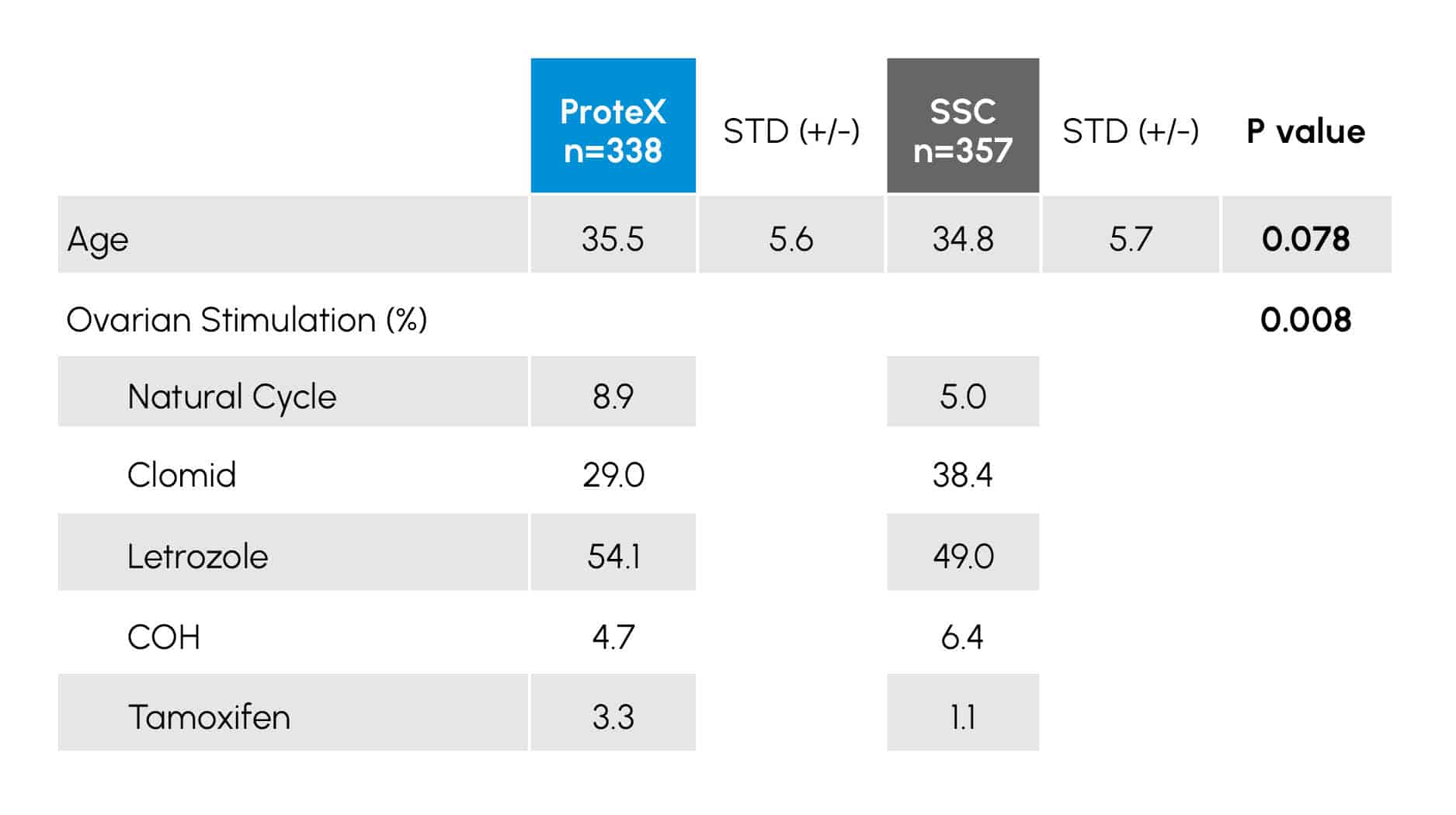
Table 2
Demographic data and stimulation Protocols of 695 women receiving semen collected in either a ProteX (n=338) or Standard Specimen Cup (SSC; n=357) for use in IUI. Data with a P value of < 0.05 are considered statistically different.
Insights
TABLE 2 provides the demographic and the data for the female patients. It was noted this was an older population with a mean age of 35.3 years. There was also a trend toward the women treated with the ProteX collected sperm to be older (P = 0.078) than those collected treated with sperm collected in the SSC.
There were differences in the stimulation regimens of the women in the study (P < .008), with more women in the SSC group receiving Clomid and more women in the ProteX going through natural cycles. Samples collected in the ProteX demonstrated higher initial volumes (P < 0.008), motilities (P < 0.001) and total motile cells (P < 0.005) compared to the SSC.
Table 3
Early outcome of IUI treatment cycles of 695 IUI cycles using either a ProteX (n=338) or Standard Specimen Cup (SSC; n=357) for semen collection. Data with a P value of < 0.05 are considered statistically different.
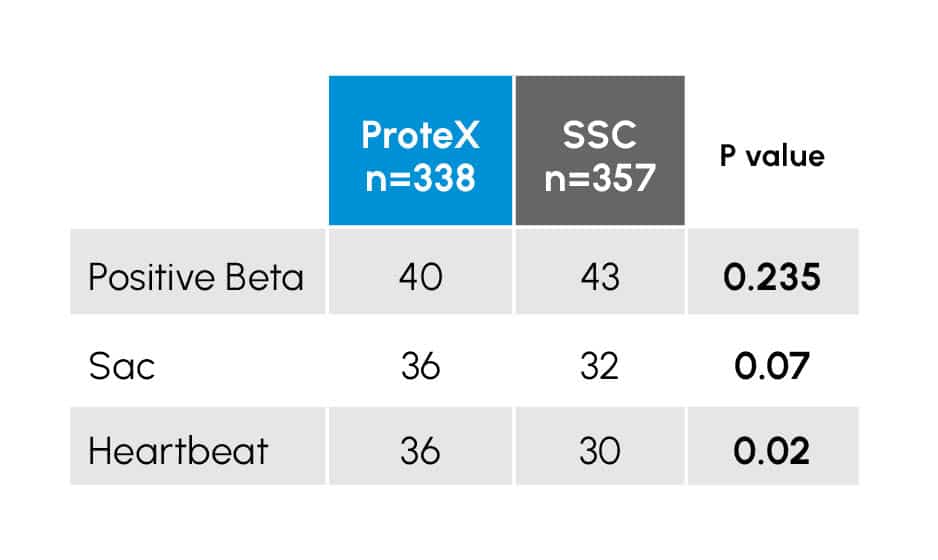
There were a total of 83 positive pregnancy tests recorded in the study population, 43 in the SSC (12.0%) and 40 in the ProteX (11.8%).
Figure 2
Early Pregnancy outcome of an IUI study comparing the ProteX to the standard specimen cup for semen (SSC) collection. Columns within an event (pregnancy test, sac development, heartbeat) with different superscripts are different at the P < 0.02 level.
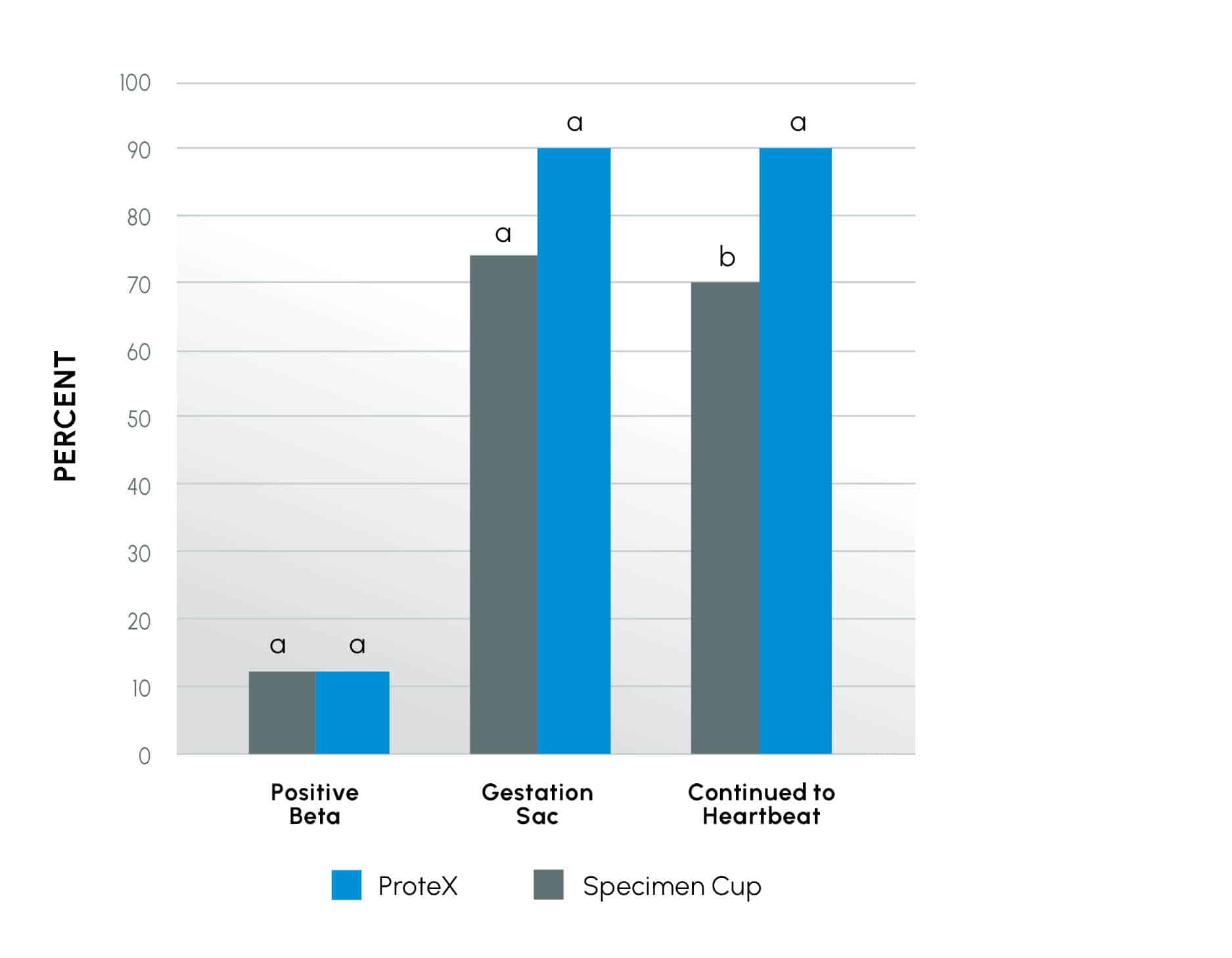
While these initial pregnancy tests were similar in both groups (P = 0.235), there was a trend (P = 0.078) toward more patients reaching sac development and a higher number of patients (up to 29% increase) in pregnancies reaching heartbeat (P < 0.02) when using the ProteX as the collection device.
Insights
Data from this study involving almost 700 patients supports earlier small-scale studies. Semen quality from samples collected in the ProteX demonstrated better initial parameters than those collected in the SSC. It is unclear why the large difference in initial volumes. However, it is possible it is the patients in the ProteX group were less stressed concerning transport times and produced better samples.
As in an earlier smaller trial, initial pregnancy data suggest more pregnancies continue on to heartbeat when the ProteX is used in the collection. Collectively these data suggest sperm collected in the ProteX may be in better physiological and biochemical condition than those collected in the SSC. This suggests healthier sperm may result in healthier pregnancies, and if all pregnancies in this trial reach delivery, a 20% higher take-home baby rate in this study.
Even with the large number of couples participating, the 83 pregnancies are still a relatively low number, and further study will be needed to confirm these results.
Direct insights into the research, methodology, and results have been added to this summary by the co-inventors themselves. This additional information is intended to provide helpful context to professional practitioners and does not fundamentally change the outcomes or interpretation of the published results. All ProteX research content and material is the property of Reproductive Solutions and may not be redistributed or republished without our consent. All rights reserved.
