Physiological and Biochemical Assessment of a New Semen Collection Device
Authors
Lisa Welch1 , Samuel Prien1 2
- Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX
- Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, Lubbock, TX
Publications
Fertility and Sterility Vol. 96, Issue 3 Supplement S163-S164. Published in issue: September 2011 – poster presentation.
Objective
Semen quality is a key factor in determining the outcome of most infertility treatments. Previously, this lab demonstrated that a modification of the collection container, known as the device for improved semen collection (DISC), resulted in significant improvement in semen quality. Studies in two animal species suggested improved semen parameters and increased pregnancy rates. The objective of the study was to assess the effectiveness of a clinical-grade version of the DISC on sperm cell function and biochemistry prior to clinical trials.
Design
A laboratory-based, controlled trial.
Materials and Methods
Nine donors supplied three samples each collected in a standard specimen cup (SSC), the DISC, or the DISC with 1 mL of media. Following collection, each sample was processed using a simple sperm washing technique and then placed in culture for 24 hours. At predetermined intervals, aliquots were taken for standard semen analysis using an IVOS and biochemical assessment, including intactness of acrosomal membranes, lipid peroxidation level, mitochondrial membrane potential, and DNA fragmentation. Resulting data were subjected to ANOVA with repeated measures.
Results
All parameters from semen collected in the DISC were either equivalent or superior to semen collected in the SSC. Specifically, samples collected in the DISC and / or DISC+ had higher rates of cell viability (p < 0.005), progressive velocity (p < 0.05) and motility index (p < 0.034) compared to the SSC and trended toward higher motility rates (p = 0.066) and path velocities (p = 0.061). Further, cells collected in the DISC had more intact acrosomes (p < 0.017) and retained higher mitochondrial membrane potential (p < 0.004) over the 24-hour period compared to the SSC.
Conclusions
Semen collected in the clinical-grade DISC appears to have superior physiological activity and biochemical stability compared to semen collected in the SSC. Clinical trials are ongoing to assess the usefulness of the DISC in the clinical environment.
Supported By
TTU Office of Commercialization.
Figure 1
Percentage of Intact Acrosomes Over Time

Percentage of intact acrosomes for spermatozoa collected in either a Standard Specimen Container (SSC), the Device for Improved Semen Collection (DISC; p < 0.017) or the Device for Improved Semen Collection containing 1 mL Ham’s F-10 media (DISC+; p < 0.009).
Insights
While standard semen analysis parameters like motility index are excellent indicators for sperm health, acrosome data is crucial for a final assessment. If the sample sperm are highly motile yet a low percentage of sperm have intact acrosome membranes, their likelihood to fertilize decreases greatly.
As in a previous acrosome study, the data here confirm samples within the DISC+ (with media) and even DISC dry, have a significantly increased number of intact acrosomes compared to the specimen cup across all time points. DISC treatments see an improvement over the specimen cup of 8% more intact acrosomes even at the initial reading.
Figure 2
Motility Index Over Time
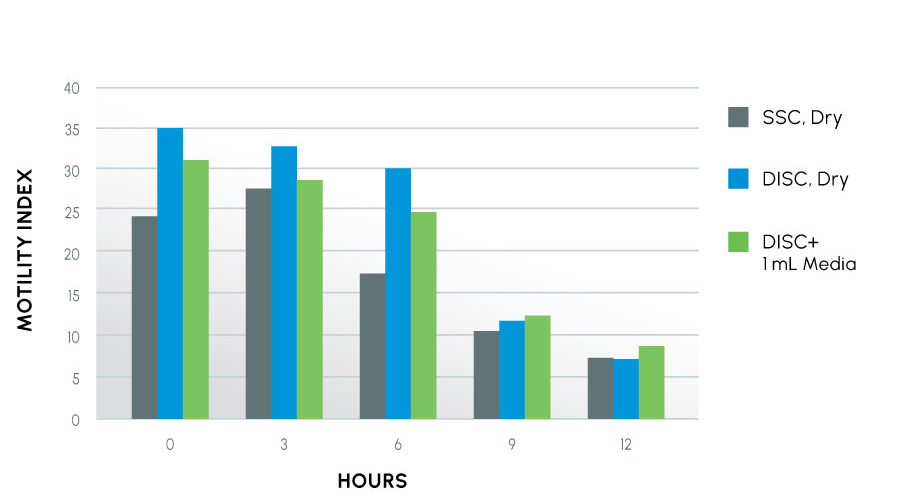
The motility index is a combination of the quantity of movement (motility) and quality of movement (progressive velocity). For this study, sperm samples were collected in three treatments by nine human volunteers without control for sample quality / subject diagnosis
Insights
Our researchers have questioned whether ProteX may be used dry and for what period of time does the sample stay viable in a dry container. In FIGURE 2 above, the results indicate the DISC dry has a higher motility index than both the SSC dry and the DISC with 1 mL media for up to 6 hours. However, coupled with the acrosome data in FIGURE 1, time points beyond 6 hours show the value of media in keeping the sample at a higher motility with more intact acrosomes.
Motile sperm, especially hyper-motile sperm, are already going through the biochemical processes needed for fertilization while also going down a parallel path to apoptosis. This process leads to the generation of cellular waste material that causes damage to the sperm over time. Research indicates the addition of media better controls production of these harmful metabolites and keeps more sperm in a quiescent state
Direct insights into the research, methodology, and results have been added to this summary by the co-inventors themselves. This additional information is intended to provide helpful context to professional practitioners and does not fundamentally change the outcomes or interpretation of the published results. All ProteX research content and material is the property of Reproductive Solutions and may not be redistributed or republished without our consent. All rights reserved.
All Scientific Studies
Sample. Test. See for yourself.
Compare ProteX to the standard specimen cup with our sterile sample kit.